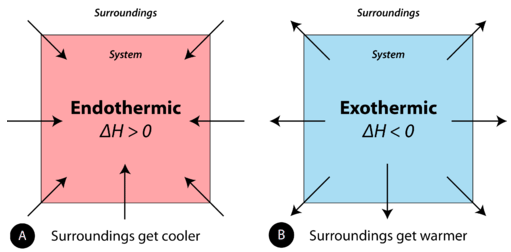
A strongly exothermic reaction will usually also be exergonic because ΔH makes a major contribution to ΔG. In an exothermic reaction the change in enthalpy ΔH will be negative.
7 3 Exothermic And Endothermic Reactions Chemistry Libretexts
The exothermic breakdown.

. The opposite is an endothermic reaction which usually takes up heat and is driven by an entropy increase in the system. Respiration transport and excretion in plants and animals. Photosynthesis is an exothermic process and cellular respiration is an endothermic process.
Understanding Endothermic and Exothermic Reactions. These exothermic and endothermic reactions. Thus respiration is an exothermic process because energy is produced during this process.
Acids bases and salts. Your muscle cells perform anaerobic respiration whenever you exhaust the oxygen being delivered to them such as during intense or prolonged exercise. Why is respiration considered as an exothermic reaction.
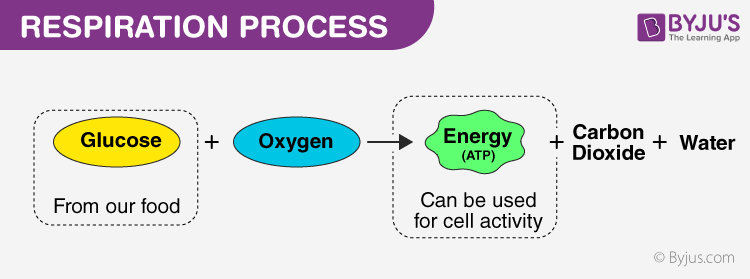
Exciting Exothermic Reactions to Try. Their definitions in terms of furnishing of H and OH ions General. Glucose and oxygen are used to release energy that organisms need to live.
Displacement precipitation endothermic exothermic reactions oxidation and reduction. The breakdown of glucose include such cellular respiration steps as glycolysis the transition reaction the Krebs cycle and oxidative phosphorylation. Anaerobic respiration is a set of chemical reactions that allows cells to gain energy from complex molecules without oxygen.
This energy can then be used to produce heat for. An endotherm from Greek ἔνδον endon within and θέρμη thermē heat is an organism that maintains its body at a metabolically favorable temperature largely by the use of heat released by its internal bodily functions instead of relying almost purely on ambient heat. In this work we investigated the use of polymer-derived SiC open-cell foams as structured.
A few examples are neutralisation burning a substance reactions of fuels deposition of dry ice respiration solution of sulphuric acid into water and much more. Tropic movements in plants. Control and co-ordination in animals and plants.
Question 6What is rancidity. The reaction in which oxygen or electronegative element is added or hydrogen or electropositive element is removed or loss of electrons takes place is. Some endothermic reactions get cold enough to cause frostbiteHeres an example of a reaction safe enough for kids to touch.
Such internally generated heat is mainly an incidental product of the animals routine metabolism but under. In human beings excretory products in the form. We can learn here about how precipitation reactions produce insoluble salts.
Heat things up with one of these simple exothermic reaction demonstrations. It involves a series of endothermic reactions. The exothermic reaction is the opposite of an endothermic reaction.
Photosynthesis is a process by which chlorophyll containing organism green. Growth and Development 6. Respiration may be aerobic or anaerobic.
How to Create an Exothermic Chemical Reaction. Aerobic respiration releases more energy as compared to anaerobic. This article throws light upon the top six processes of plant physiology.
Respiration is the chemical change that takes place inside living cells. Both photosynthesis and cellular respiration store energy from the Sun in the form of glucose. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction.
IPotassium bromide aq Barium iodide s iiZinc carbonate s - Zinc oxide s Carbon dioxide g. The products in the equation for photosynthesis are the reactants in the equation for cellular respiration. It releases energy by light or heat to its surrounding.
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Respiration is a series of exothermic reactions that occur in the mitochondria of living cells in order to release energy from food molecules. Download CBSE Class 10 Science Syllabus 2022-2023 in PDF here.
Create an Endothermic Reaction. Our online biochemistry trivia quizzes can be adapted to suit your requirements for taking some of the top biochemistry quizzes. The introduction with the example of exothermic and endothermic reactions are also important for board exams.
4 stages of cellular respiration are metabolic pathways that contribute to the production of ATP molecules in cells. A comprehensive database of more than 83 biochemistry quizzes online test your knowledge with biochemistry quiz questions. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic.
C6H 12 O 6aq 6O 2g 6CO 2g. Check the revised syllabus and read the chapters topics mentioned in this latest syllabus only. The catalytic methanation of CO2 via the strongly exothermic equilibrium Sabatier reaction requires the development of structured catalysts with enhanced mass- and heat-transfer features to limit hot-spot formation avoid catalyst deactivation and control process selectivity.
Question 7Write the balanced chemical equation of the following and identify the type of reaction in each case. Is oxidation an exothermic or an endothermic reaction.

Difference Between Endothermic And Exothermic Reactions Chemistry

Question Video Recognizing That Aerobic Respiration Is An Exothermic Reaction Nagwa

Because Respiration Requires Energy Is It Endothermic Quora
Why Is Respiration Considered An Exothermic Reaction Explain Science Question
0 Comments